Participating Sites FAQs
Many questions may arise while you are recruiting and screening patients and when doing study visits. Here are the answers to some of the more common questions we have had. If the answer to your question isn’t here, or you are still unsure, please don’t hesitate to contact the study team at reduce2@bsms.ac.uk
Background and Rationale:
- What is the significance of refractory ascites in patients with advanced cirrhosis?
Refractory ascites is a serious complication of advanced cirrhosis, leading to discomfort and shortness of breath due to repeated accumulation of fluid in the abdomen. The only cure is a liver transplant, but many patients are not eligible for this. Therefore, refractory ascites can be a hallmark of end-stage liver disease with a median survival of 6-12 months.
- Why is refractory ascites associated with a high symptom burden?
Refractory ascites, and the repeated accumulation of fluid in the abdomen, causes distension and pressure on surrounding organs. This results in pain and breathlessness due to the restricted movement of the diaphragm and increased pressure on the lungs. It can also affect patients’ appetite and restrict mobility due to the weight of the fluid.
- What is the current standard of care for patients with refractory ascites?
A liver transplant is the only cure. TIPS (Transjugular Intrahepatic Portosystemic Shut), which is a procedure that drains the fluid by allowing blood to bypass the stiff, scarred liver through a shunt, can improve survival. For many patients however, neither is a realistic option, and the standard of care remains repeated drainage through a procedure known as large volume paracentesis (LVP). This is usually performed every two to three weeks in a hospital setting. With up to 10-12 litres of fluid removed at each visit. To avoid significant fluid shifts and to protect the kidneys, human albumin (protein) solution is given through a cannula during this procedure.
- What is a long-term abdominal drain (LTAD) and how can they help patients with refractory ascites?
LTAD is a tunnelled drain which is inserted in the abdominal wall under local anaesthetic in hospital. The tunnelled portion allows for the drain to be held in place, while community nurses or informal caregivers drain small amounts (1-2 litres) of ascitic fluid at home, up to three times a week. LTADs have potential advantages over the standard of care as they could reduce hospitalisation, improve symptom control and health related quality of life.
- Why are they not used already for patients with cirrhosis?
LTADs are currently recommended by the National Institute for Clinical Excellence (NICE) for malignant ascites. However, in cirrhosis and liver disease there is not enough evidence to support their use, with concerns of complications such as infection, bleeding, and kidney problems. A feasibility study showed they are potentially safe, but more research is required. NICE currently recommends that LTADs should only be placed in the context of research or heavily audited practice.
Study Aim:
- What are the primary and secondary outcomes of this study?
The primary aim of the study is to evaluate whether palliative LTADs improve the health-related quality of life (HRQoL) of patients with refractory ascites due to advanced cirrhosis compared to the current standard of care, Large Volume Paracentesis (LVP). The primary outcome is the short form liver questionnaire (SF-36) assessed 4-weekly, while secondary outcomes will look at caregiver workload, infection rates, health resource utilisation and symptom burden.
Eligibility and screening:
- Are patients with hepatocellular carcinoma (HCC) eligible for the study?
Yes. HCC is not an exclusion criteria and patients with suspected or confirmed HCC can still take part if they meet the rest of the inclusion criteria. Just make sure HCC and any history of treatment is added to the eCRF.
- Can patients with both heart failure and cirrhosis be recruited to the study?
Yes, heart failure is not an exclusion criterion. If a patient has cirrhosis, irrespective of the aetiology, and it is felt that it is a significant contributing factor for the ascites, then they are eligible for the study. Please discuss any such patient with your local PI to confirm eligibility and if there is uncertainty following discussion with the PI, please email the trial team with details.
- Can patients who do not speak English take part in the study?
Many of the study outcomes are based on questionnaires that have only been validated in English. However, given that our protocol allows for ‘proxy’ scores to be used, patients who do not speak English can be recruited if they have an informal caregiver who can translate the questions and act as an interpreter. We have funding available to translate the Patient Information Sheet (PIS) and consent forms to other languages in those cases. Please get in touch sooner rather than later with the trial team as the turnaround time for issuing and approving the translated documents can take several weeks.
- What if a patient is out of area?
For tertiary centres taking part in the study this might be an issue. Sites must serve the community trust where the patient is registered with a GP. This will ensure direct communication and a link with district nurses performing the drainage. If you identify a suitable patient who is out of area please liaise with the study team, and we can forward their details to the PI of the local site if they’re taking part in REDUCe2.
- Are patients eligible if they are part of another study?
Being part of another study is not an exclusion criterion for REDUCe2 if there is no conflict between the two protocols. In some studies (for example ASEPTIC), the requirement for antibiotic prophylaxis can affect the follow-up of patients already recruited to ASEPTIC. Following discussion with the CI of ASEPTIC, patients already recruited to ASEPTIC cannot be recruited to REDUCe2 and should stay on the ASEPTIC trial until their final follow-up date.
Consent and Randomisation:
- Do I need to repeat an ascitic tap if one was done recently at the time of screening?
Not necessarily. The window for each visit is +/-3 days, and a negative tap (as per protocol) from within that window can be sufficient for randomisation. Just remember that this might limit the available window (10 days) for the LTAD insertion before re-screening is required (see below).
- Can research nurses consent patients into REDUCe2?
Yes, if the nurses have been trained to do so and are authorised by the PI having signed the appropriate duties in the delegation log.
- How do I randomise a patient?
Access to Sealed Envelope can be granted for team members who will be randomising participants. The participant must be registered on Ennov eCRF (and a unique study number generated) before being randomised. Sealed Envelope will ask for this number along with the participant’s date of birth, Child Pugh Score and sex before they are assigned a group.
- What if the patient is randomised to the standard of care arm?
This can be a disappointing outcome for the participant, caregiver, and researcher alike however with good counselling as part of study initial consideration it is not insurmountable. We will be providing communication skills videos that might help in this situation. Some patients may wish to withdraw from the study after hearing this. However, it is important to reiterate the invaluable data they would be providing as controls in this trial. Some patients value the extra input they get from the fortnightly research visits and it’s important for them to realise that they can opt to have the LTAD inserted outside the trial setting after 3 months study follow-up has been completed.
Baseline Visit and LTAD Insertion:
- What if the LTAD insertion date is cancelled?
To ensure that an LTAD can be inserted safely and provide any necessary blood products, the procedure must be performed within 10 days of the date that a negative ascitic tap was taken and bloods (such as clotting and full blood count) were done. They must meet the criteria set in the protocol. If the planned LTAD insertion is cancelled there is no need to re-randomise, but there may be a need for re-screening depending on the timeline. Re-screening will involve a repeat ascitic tap and blood tests. The baseline visit might also need to be delayed so that it falls +/- 3 days of the insertion of the first LTAD.
- My patient randomised to the LVP arm does not need a drain for a couple of weeks, when can we do the baseline visit?
The timings of LVP run completely independently to the timings of the visits. Ideally the baseline should be done within 7+/-3 days of the screening visit as per protocol regardless of the timing for the next drain. But we appreciate that a home visit for this might not be feasible and bringing in the patient in for an extra hospital visit should be avoided if possible. The baseline visit can therefore be delayed until the patient comes into hospital for their next LVP in exceptional circumstances and would be a protocol deviation.
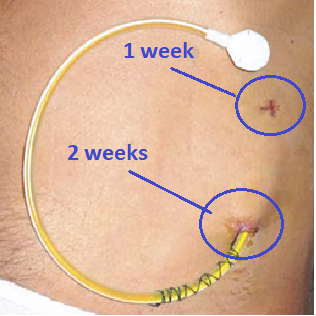
- When should the sutures for the LTAD be removed?
The deep suture approximately 5 cm down where the drain is tunnelled should be removed after 1 week, whereas the suture next to the drain insertion should be left in situ for at least 2 weeks. They are therefore expected to be in place at the W2 visit and can be removed if the wound has healed and there are no concerns about infection.
- A patient’s LTAD fell out/got blocked and needs replacing during the study period, do they need to have another baseline visit?
No this would not ‘reset the clock’. The visits should continue as planned (i.e. two weekly from the successful insertion date of the first LTAD).
- How much should be drained on the day of insertion of the LTAD?
Immediately after insertion of the LTAD, draining as much ascites as possible (with HAS infusion as per LVP protocols) is recommended to reduce the chance of subsequent cellulitis and leakage around the drain site. This makes the ascites management in the community is easier. We recommend using standard LVP volume limits in high-risk patients i.e. 6-8L in those with previous circulatory dysfunction, HRS or chronic kidney disease. There is no need to bring the patient back in for another large volume drain, and community drainage can continue as per usual.
- Does the IR team need to be GCP trained?
No, the drain insertion is standard of care (as it’s done for patients with malignant ascites). The IR team do not need to be GCP trained or added to the delegation log.
- Do I need to refer to the Palliative Care team?
We are aware that not all patients will have immediate palliative care needs, however, patients with refractory ascites have a limited life expectancy and one of the aims of REDUCe2 is to improve the links between hospital care and community palliative input for this cohort. We also appreciate the immense pressure our palliative care colleagues are under, and that not all referrals will be picked up. We will be monitoring the outcome of these referrals and you do not need to chase it once it’s been made unless the participant subsequently develops palliative care needs.
- Which antibiotic prophylaxis should we use?
This will depend on your local trust policy for primary/secondary prophylaxis against spontaneous bacterial peritonitis (SBP). However, for some patients (especially those already on antibiotics for another indication), a discussion with microbiology is warranted. Patients and caregivers should be counselled about the risks and benefits of antibiotic prophylaxis.
- Do they still need it if they’re on rifaximin?
Yes as this might not be sufficient to reduce the risk of SBP in the presence of an indwelling drain.
- Are the antibiotics funded as a research medication?
No. This has been costed as a SOC treatment. To avoid introducing bias and standardise practice across 35 sites nationally we have incorporated this into the protocol since some sites start patients at high risk of SBP on primary prophylaxis as standard of care (e.g. low ascitic protein, refractory ascites with repeated drains). Patients in the LVP group must be put on antibiotic prophylaxis for the duration of the trial, to ensure consistency and comparability with the LTAD group.
Study Visits:
- Can the LTAD patient travel to hospital for completion of questionnaires?
Given our primary outcome is health related quality of life, and reducing hospital visits can impact this, bringing the patient in to complete study related questionnaires could bias the results. We therefore ask that no extra hospital visits are scheduled for either group for the purpose of the study. Questionnaires can be completed at hospital if the patient is already attending for an LVP, any other routine appointment or has been admitted.
- Do research visits need to be completed by a nurse/doctor?
No. Anyone can do the visits if they are adequately trained, have completed GCP and are on the delegation log. Agreements should be made locally to ensure clear links with the PI for any clinical questions that might arise.
- What happens if the patient loses capacity during the study?
For sites in England and Wales, an agreement should have been made at the time of initial consent for a named consultee who can be approached in this situation. This can be an informal caregiver (personal consultee) or an independent medical professional (nominated consultee). Try and complete as many questionnaires as possible (with the aid of the proxy version questionnaires if need be).
- Who needs to fill out the LTAD drain diaries?
As these diaries are part of a patient’s official medical record, they should only be filled out by the community nurses. If a caregiver is performing some of the drainage some of the time, then this should be transcribed onto the diaries by an appropriately trained medical professional.
- How are drainage bags/bottles disposed of?
This varies across regions and councils. Please follow your local processes.
Questionnaires and data entry:
- Who needs to complete the Modified AHCR?
This can be filled by a participant/caregiver or healthcare professional. Some sections are not relevant if the participant does not have a caregiver and can be skipped.
- How often do I need to enter the data onto Macro and the SHORE-C database?
Ideally visit data should be entered within two weeks of the visit.
- Can proxy scores be used?
Yes, especially if the patient has lost capacity and is unable to answer some of the questions. Some of the questionnaires have proxy versions. Please make a note on the questionnaires that proxy scores were used.
- Which questionnaires do I enter where and which ones do I send?
Liver Disease QoL (SFLDQoL) questionnaire (completed by the patient) and the Caregiver Roles & Responsibilities Scale (CRRS) and CRRS About You (completed by the Caregiver at baseline, if applicable) should be entered on to the SHORE-C database. Please use this link: shore-c.sussex.ac.uk/reduce/reduce
The SFLDQoL, CRRS and CRRS About They should then be emailed to SHORE-C for Quality Control – shorec-reduce2@sussex.ac.uk.
The consent and expression of interest forms need to be emailed to the study team (reduce2@bsms.ac.uk) before caregiver data can be entered.
All other data and questionnaires should be entered on to the MARCO eCRF.
- What should I do if I don’t have blood tests results to complete the liver disease scores?
You can use blood results from an earlier test (but NOT from the previous visit). If there are no recent blood results, please right-click on the field and mark as not available.
- What to do if there is an SAE?
Only serious events that are related to the drains (Serious Adverse Reaction) need to be reported to the CTU, but please record all events in the eCRF.
End of the Study:
- Can those in the LVP group receive an LTAD at the end of the study?
Yes. However, the risks, benefits, and uncertainties surrounding LTADs must be explained to the patient and clearly documented. LTADs outside the trial setting would not be provided by the study team, and the process for ongoing support/follow-up of these patients needs to be put in place locally.
- Can those in the LTAD group keep it in place at the end of the study?
Yes, their care should be handed to the community teams as well as their usual gastroenterologist and an ongoing supply of drainage bags/bottles needs to be ensured by General Practitioners. We also recommend that patients continue antibiotic prophylaxis for as long as the drain is in place.
- How long can patients keep the drain in for?
There is no set expiry date for the LTAD, and it is worth having a pragmatic approach with the patient given its palliative intent. It can be kept in as long as it’s functioning with no issues. Although it is worth mentioning that some reports suggest increased complications with the drain after 6 months.
Study Supplies & Laboratory Samples:
- Do sites receive LTAD drainage kits or do they need to use their own hospital stock?
For the duration of the study, sites will receive either Rocket Medical IPC Indwelling Peritoneal insertion kits with drainage bags or BD PeritX™ Peritoneal Catheter Systems with drainage bottles depending on their preference. Additional kits will be supplied by both manufacturers once a patient has been randomised to the LTAD arm.
- What else will sites be supplied with?
Sites will receive 2 x blood kits for the optional research blood sample taken at baseline along with a cryobox, cryovials and labels for the frozen samples. Sites will also be sent the LTAD Drainage Diaries on no-carbon required paper and LVP diaries are sent via email.
- How do I reorder more supplies?
Additional blood kits can be re-ordered from UHSussex Research Laboratory by emailing Dominika.Wlazly@nhs.net. Additional LTAD Drainage Diaries can be ordered from reduce2@bsms.ac.uk.
- What happens to the frozen samples at the end of the study?
The Trial Manager will arrange for a courier with dry ice to collect the frozen samples after liaising with the sites.
Finances:
- What’s the process for raising invoices?
The Trial Manager will request a PO number to be raised every quarter. Invoices will then need to be issued to University Hospitals Sussex NHS Foundation Trust as they hold the study grant money. Any invoice questions should be directed to uhsussex.research.accounts@nhs.net.
- Can you confirm the travel expenses for patients?
Patients on the LTAD arm can claim reasonable travel expenses (up to £30) for the Baseline visit only. Receipts should be obtained from the patient wherever possible. The Trial Manager will ask if any travel expenses need claiming for before raising the PO.
- Can you clarify the staff travel expenses for the research home visits?
Staff can claim travel expenses for the fortnightly home visits, up to a maximum of £100 per patient for up to 6 visits. Receipts should be kept wherever possible. The Trial Manager will ask if any travel expenses need claiming for before raising the PO.
- What if the travel costs are more than the cut-off mentioned?
Since not all participants will require six home visits for various reasons, we hope on average that the £100 would be enough. There is a pot of money (1k) that is available for each site to engage with community teams, and some of that can go towards any excess costs.